
About Us
Chemo Dynamics is New Jersey based CRO facility with GMP (Good Manufacturing Practice) infra-structure and have kilo laboratories including a pilot plant. We undertake custom synthesis and small-lot manufacturing. We provide all types of services related to small molecule synthesis. We simply focus on doing appropriate chemistry for the clients emphasizing Science Simplicity, Product Innovation, and Rigorous Safety.
Our goal, motto, and attitude are to ensure cost-efficient and timely services following ICH guidelines.
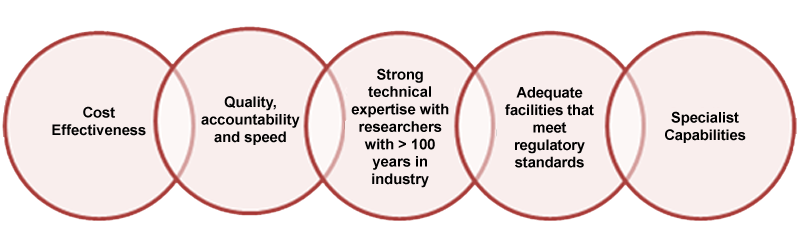
We have 60+ Years of Experience to provide Superior Quality Results.
How do we manage your Project – 6 Step Approach
Step 1
Identify Customer’s Goals and Objectives.
Enquire Now ↬

Step 2
Execute appropriate confidentiality and non-disclosure agreements to ensure IP protection.
CDA template
NDA template

Step 3

Step 4
Identify appropriate research/platform from which to execute a project. Typically Chemodynamics uses Patents, Literature, Sci-finder, Notebook Procedures, etc.

Step 5
Undertake appropriate
analytical procedures and
quality control at each
phase of the project
execution. Will generate a suitable template starting from POC(proof of concept) sample template.
Sample POC Template

Step 6
Deliver product to customer and allow for review and further adjustments, if necessary.
Our Vision
Our Mission
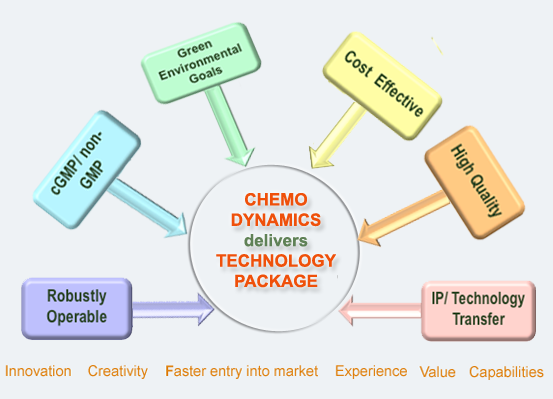
To help clients with our CRO services by delivering secure, effective, and faster entry into the market, providing quality assurance beyond SOP, and earning trust, reliability, and confidence in quality and integrity.
Our mission is to be a part of your outsource team, providing high-quality custom synthesis and process development while minimizing time to market and helping you to balance and control costs.


When you choose a vendor to handle your most challenging chemistries, think of a first-rate company with a reputation for doing a high-quality job.
Think of Chemo Dynamics!
ChemoDynamics is an experienced, contract development and manufacturing company for active pharmaceutical ingredients (API) and pharmaceutical intermediates that combines the benefits of working with a contract research organization (CRO) and a contract manufacturing organization.
Business Model
We Offer a Broad Spectrum Of Services Including:
Custom non-GMP Synthesis
Custom
cGMP
Synthesis
Route
Scouting
Scale up and Kg Scale Production
Contract Research
&
Manufacturing
Bench Scale Process Development
Stability
Studies
Analytical Method Development
Business Model
We Offer a Broad Spectrum Of Services Including:
Custom non-GMP Synthesis
Custom
cGMP
Synthesis
Contract Research
&
Contract Manufacturing
Bench Scale Process Development
Stability
Studies
Analytical Method Development
Scale up and Kg Scale Production
Route
Scouting
Plan For Growth


Our Core Values
Professionalism
We proudly exhibit a consistent commitment to service quality and efficiency in all our work.
Integrity
We work with the highest standards of integrity and honesty.
Accountability
We are accountable for what we say and do. We say what we mean and do
Creativity
We approach challenges with creativity and flexibility. We constantly search for ways to improve our services and create more value for our clients.
Respect
We respect the IP of our clients and protect CDA/NDA.
Our STAFF is composed primarily of Ph.D. Chemists who are not only talented at the bench but ready to discuss and recommend alternate ways to do your job.
Our team adheres to strict confidentiality protocols and delivers a broad spectrum of SERVICES, including:
- Bench and kilogram scale synthesis
- Process research
- Analytical development
Leveraging our state-of-the-art GC-MS, LC-MS & NMR equipment to serve existing and new customers ensures RESULTS that are safe, operable, and economical. We ensure that our clients have the information and the quantity of material they need to take their projects to the next level.

Services Offered
We provide products and services to global pharmaceutical companies, biotechnology companies, and government agencies worldwide to bring drugs to market faster and more efficiently. Our portfolio of products and services enables our customers to reduce costs, increase speed to market and enhance their productivity and effectiveness in drug discovery and development.
Our broad spectrum of services includes Custom synthesis, Contract R&D, Route selection, Process research/scale-up, Analytical development, impurity isolation, identification & synthesis, and Small lots manufacturing. We also provide cGMP services and have a Schedule II – V Controlled Substance License.
Background
- 1960 Chemo Dynamics was incorporated
- 1998 Dixie takes over; Facilities upgraded
- 2000 cGMP Suites completed
- 2009 Under new ownership
- 2011 Major upgrade of facilities
Our Facility












Equipment
Our work culture

Reliability

Quality

Innovation

Timing

IP-Protection



